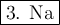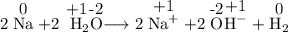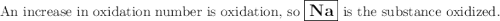Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, Averybeam300
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 22.06.2019 06:00, citlalli30
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 12:10, coastieltp58aeg
Building glycogen from glucose molecules is an example of
Answers: 3

Chemistry, 22.06.2019 15:00, alanmarcus22
What does the symbol (–hfus) indicate in a phase change?
Answers: 1
Do you know the correct answer?
Given the reaction: 2na + 2h2o → 2na+ + 2oh− + h2
which substance is oxidized?
1.
...
1.
...
Questions in other subjects:

English, 21.10.2019 21:30

Biology, 21.10.2019 21:30


English, 21.10.2019 21:30


Mathematics, 21.10.2019 21:30



English, 21.10.2019 21:30

Mathematics, 21.10.2019 21:30