
Chemistry, 12.06.2020 12:57, milkshakegrande101
. 125g of water has an initial temperature of 25.6°C, and is heated by 50.0g of a metal
which has been heated to 115.0°C. The metal heats the water so that both the metal
and the water reach a final temperature of 29.3°C. Calculate the specific heat of the
metal.


Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:00, Killion2022
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 15:30, 20cschultz
Which suspect most likely committed the robbery and how do you know
Answers: 2


Chemistry, 22.06.2019 20:40, oddoneshenchman
Why do lunar and solar eclipse not happen every month
Answers: 2
Do you know the correct answer?
. 125g of water has an initial temperature of 25.6°C, and is heated by 50.0g of a metal
which has b...
Questions in other subjects:



Social Studies, 18.12.2020 17:50



Computers and Technology, 18.12.2020 17:50

Mathematics, 18.12.2020 17:50

Mathematics, 18.12.2020 17:50





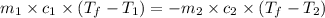
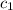 = specific heat of metal = ?
= specific heat of metal = ?
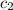 = specific heat of water =
= specific heat of water = 
 = mass of metal = 50.0 g
= mass of metal = 50.0 g
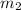 = mass of water = 125 g
= mass of water = 125 g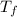 = final temperature of mixture =
= final temperature of mixture = 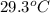
 = initial temperature of metal =
= initial temperature of metal = 
 = initial temperature of water =
= initial temperature of water = 
![(50.0g)\times c_1\times (29.3-115.0)^oC=-[(125g)\times 4.18J/g^oC\times (29.3-25.6)^oC]](/tpl/images/0684/0377/2c651.png)





