Chemistry, 12.06.2020 05:57, SassyChumpkins
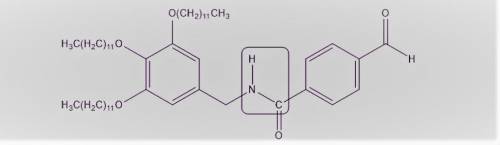
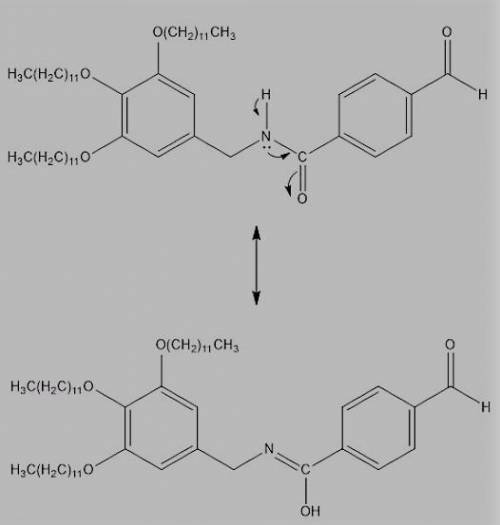
In Chapter 4, we will learn that single bonds experience free rotation at room temperature, while double bonds do not. Consider the two C-N bonds in the structure. One of these bonds exhibits free rotation, as expected for a single bond, but the other C-N bond exhibits restricted rotation. Identify the C-N bond with restricted rotation, and justify your answer by drawing resonance structures.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, anamaliiow
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 03:50, AysiaRamosLee
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 08:00, ggdvj9gggsc
Asap! will give brainiest when a heat wave strikes a region causing more people to run air-conditioning units, electrical demand increases. what needs to be done to meet this increased demand? raising the control rodslowering the control rodsremoving the control rods
Answers: 1
Do you know the correct answer?
In Chapter 4, we will learn that single bonds experience free rotation at room temperature, while do...
Questions in other subjects:

Mathematics, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

History, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01

English, 17.09.2020 08:01

Mathematics, 17.09.2020 08:01