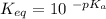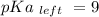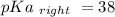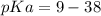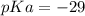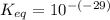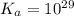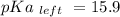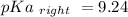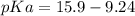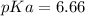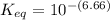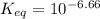Chemistry, 12.06.2020 04:57, katekayrodriguez10
Calculate Keq for these reactions and predict if the equilibrium will lie to the right or to the left as written. (You may enter your answer in scientific notation, e. g. 1.0*10^-9. Enter your answer to two significant figures.) Reaction 1: + + pKa = 9 pKa = 38 Keq = Equilibrium position = Reaction 2: + + pKa = 35 pKa = 25 Keq = Equilibrium position =

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, mastershadow2018
Agroup of students is studying convection currents. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other in an area with warm air. after 10 minutes, the balloons are released from a height of 1 meter. which of the following do the students most likely observe? a. the balloons both rise. the cold balloon is larger than the warm balloon. b. the balloons rise at the same rate. both balloons are the same size. c. the warm balloon expands and rises. the cold balloon shrinks and sinks. d. the cold balloon expands and rises. the warm balloon shrinks and sinks.
Answers: 2


Chemistry, 22.06.2019 22:10, steven0448
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
Do you know the correct answer?
Calculate Keq for these reactions and predict if the equilibrium will lie to the right or to the lef...
Questions in other subjects:




Mathematics, 09.02.2021 16:20

Chemistry, 09.02.2021 16:20

History, 09.02.2021 16:20



Mathematics, 09.02.2021 16:30


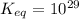
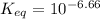
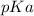 is mathematically evaluated as
is mathematically evaluated as 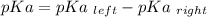
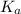 is mathematically evaluated as
is mathematically evaluated as