Chemistry, 12.06.2020 02:57, lilinicholeb
From the unbalanced reaction: B2H6 + O2 ---> HBO2 + H2O
How many grams of O2 (32g/mol) will be needed to burn 36.1 g of B2H6 (Molar mass = 27.67g/mol)? g
Include the correct number of significant figures in your final answer

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:30, jamesnaquan132
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 13:30, kkingstone7062
What does the xylem do? stores the glucose captures the sunlight absorbs oxygen into the leaf carries water from the roots to the leaves
Answers: 1

Chemistry, 22.06.2019 19:30, simihehe
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
Do you know the correct answer?
From the unbalanced reaction: B2H6 + O2 ---> HBO2 + H2O
How many grams of O2 (32g/mol) will be n...
Questions in other subjects:


Mathematics, 05.05.2020 12:33

English, 05.05.2020 12:33








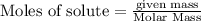
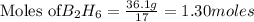
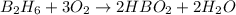
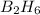 require = 3 moles of
require = 3 moles of 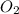
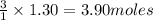 of
of