
The half-life of radium-226 is 1590 years. (a) A sample of radium-226 has a mass of 50 mg. Find a formula for the mass of the sample that remains after t years. (b) Find the mass after 500 years correct to the nearest milligram. (c) When will the mass be reduced to 40 mg

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:30, Sumitco9578
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 23.06.2019 06:20, cowboo5000pcl655
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1

Chemistry, 23.06.2019 06:30, jamarstand
Which of the following steps is not likely to take place during cellular respiration? (5 points) select one: a. oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. c. simple sugar breaks down. d. energy is used up.
Answers: 1
Do you know the correct answer?
The half-life of radium-226 is 1590 years. (a) A sample of radium-226 has a mass of 50 mg. Find a fo...
Questions in other subjects:


English, 08.12.2021 03:50

Advanced Placement (AP), 08.12.2021 03:50

Computers and Technology, 08.12.2021 03:50


Social Studies, 08.12.2021 03:50

History, 08.12.2021 03:50



Mathematics, 08.12.2021 03:50


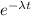
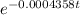
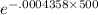
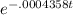
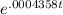 = 1.25
= 1.25 



