
In E. coli, the enzyme hexokinase catalyzes the reaction: Glucose + ATP → glucose 6-phosphate + ADP The equilibrium constant, Keq, is 7.8 x 102. In the living E. coli cells, [ATP] = 7.9 mM; [ADP] = 1.04 mM, [glucose] = 2 mM, [glucose 6-phosphate] = 1 mM. Determine if the reaction is at equilibrium. If the reaction is not at equilibrium, determine which side the reaction favors in living E. coli cells.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:30, madmatt873
What volume of a 0.0606 m solution of strontium bromide is needed to obtain 0.340 mol of the compound? question 42 options: a)5.61 l b) 3.4 l c) 600 ml d) 1 l e) 178 ml
Answers: 1


Chemistry, 23.06.2019 11:00, lildestinyquintana
Acompound is isolated from the rind of lemons that is found to be 88.14% carbon and 11.86% hydrogen by mass how many grams of c and h?
Answers: 2

Chemistry, 23.06.2019 15:20, masonrochester7
How many stereoisomers will be formed from the addition of phenyllithium to this molecule?
Answers: 1
Do you know the correct answer?
In E. coli, the enzyme hexokinase catalyzes the reaction: Glucose + ATP → glucose 6-phosphate + ADP...
Questions in other subjects:


Mathematics, 10.09.2019 00:20


Physics, 10.09.2019 00:20

Mathematics, 10.09.2019 00:20

Mathematics, 10.09.2019 00:20

History, 10.09.2019 00:20

Mathematics, 10.09.2019 00:20

Mathematics, 10.09.2019 00:20


![q=\frac{[\text {glucose 6-phosphate}][ADP]}{[Glucose][ATP]}](/tpl/images/0683/3692/46a36.png)
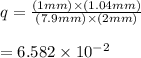
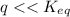 ⇒ following this criteria the reaction will go towards the right direction ( that is forward reaction is favorable until q = Keq
⇒ following this criteria the reaction will go towards the right direction ( that is forward reaction is favorable until q = Keq




