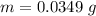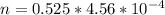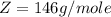Chemistry, 10.06.2020 19:57, jerseygirl3467
At 25.0 °C the Henry's Law constant for sulfur hexafluoride (SP) gas in water is 2.4x 10 M/atm Calculate the mass in grams of SFo, gas that can be dissolved in S25. ml. of water at 25.0 C and a SF, partial pressure of 1.90 atm Be sure your answer has the correct number of significant digits.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:50, kukisbae
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 04:30, logan12345677885675
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2


Chemistry, 22.06.2019 14:30, cxttiemsp021
Calculate the mass of carbon in 97.0 g of sucrose c12h22o11
Answers: 3
Do you know the correct answer?
At 25.0 °C the Henry's Law constant for sulfur hexafluoride (SP) gas in water is 2.4x 10 M/atm Calcu...
Questions in other subjects:

History, 02.12.2020 19:50


History, 02.12.2020 19:50

English, 02.12.2020 19:50






Mathematics, 02.12.2020 19:50