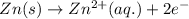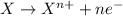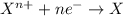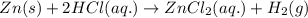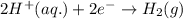Chemistry, 10.06.2020 03:57, willveloz4
Suppose 5.00g of Zn metal is completely consumed in an HCl solution to produce zincil) choride (ZnCl2) and
hydrogen gas (H2) according to the following reaction:
Zn(s) + 2HCl(aq) > ZnCl2(aq) + H2(g)
Part A
How many moles of ZnCl2 are produced?
moles =
Part B
How many grams of H2 are produced?
grams =

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, mvtthewisdead
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1


Do you know the correct answer?
Suppose 5.00g of Zn metal is completely consumed in an HCl solution to produce zincil) choride (ZnCl...
Questions in other subjects:

Biology, 09.12.2020 07:50


Arts, 09.12.2020 07:50

Mathematics, 09.12.2020 07:50

Computers and Technology, 09.12.2020 07:50

Mathematics, 09.12.2020 07:50


Mathematics, 09.12.2020 07:50

Mathematics, 09.12.2020 07:50

Biology, 09.12.2020 07:50