
Chemistry, 09.06.2020 19:57, josephicarusmarrujo
At high temperatures one mole of hydrogen gas reacts with one mole of bromine gas to form hydrogen bromide. At a given temperature the equilibrium constant is 57.6. If at the same temperature, a mixture of 4.67 × 10^-3M bromine gas, 2.14 × 10^−3 hydrogen gas, and 2.40 × 10^−2M hydrogen bromide gas is made, then:
a. the system is at equilibrium.
b. the system is far from equilibrium and will shift to form more hydrogen gas.
c. the system is far from equilibrium and will shift to form more hydrogen bromide gas.
d. nothing can be deduced since we do not know whether the reaction is endothermic or exothermic.
e. nothing can be deduced since we do not know whether the equilibrium constant is Kc or Kp.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:10, jasondesatnick
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 14:10, roserose3098
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 15:30, elizabethprasad2
The reactions of photosynthesis occur in the of plant cell? a. mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
Do you know the correct answer?
At high temperatures one mole of hydrogen gas reacts with one mole of bromine gas to form hydrogen b...
Questions in other subjects:


Chemistry, 02.11.2020 08:20


Mathematics, 02.11.2020 08:20

Mathematics, 02.11.2020 08:30


Mathematics, 02.11.2020 08:30

Mathematics, 02.11.2020 08:30


Biology, 02.11.2020 08:30


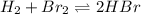
![Keq=\frac{[HBr]^2}{[H_2][Br_2]} =57.6](/tpl/images/0680/9707/6b896.png)
![Q=\frac{[HBr]^2}{[H_2][Br_2]} =\frac{(2.40x10^{-2})^2}{(4.67x10^{-3})(2.14x10^{-3})} \\\\Q=57.6](/tpl/images/0680/9707/53e8b.png)




