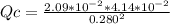Consider the following reaction where Kc = 1.80×10-2 at 698 K:
2HI(g) → H2(g) + I2(g)
A reaction mixture was found to contain 0.280 moles of HI (g), 2.09×10^-2 moles of H2 (g), and 4.14×10^-2 moles of I2 (g), in a 1.00 liter container.
Required:
a. Is the reaction at equilibrium?
b. What direction must it run in order to reach equilibrium?
c. The reaction
1. must run in the forward direction to reach equilibrium.
2. must run in the reverse direction to reach equilibrium.
3. is at equilibrium.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:10, Rubendelarosa1529
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3

Chemistry, 23.06.2019 02:30, hailee232
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1

Chemistry, 23.06.2019 08:00, LuvIsRage2
Technician a says that you should never jump-start a frozen battery. technician b says that a frozen battery can explode, causing injury, when jump-started. who is correct?
Answers: 2
Do you know the correct answer?
Consider the following reaction where Kc = 1.80×10-2 at 698 K:
2HI(g) → H2(g) + I2(g)
...
...
Questions in other subjects:







Mathematics, 19.10.2021 01:30



Mathematics, 19.10.2021 01:30


![Qc=\frac{[C]^{c}*[D]^{d} } {[A]^{a}*[B]^{b}}](/tpl/images/0680/9750/5af8a.png)
![Qc=\frac{[H_{2} ]*[I_{2} ] } {[HI]^{2}}](/tpl/images/0680/9750/8091f.png)
![[H_{2} ]=\frac{2.09*10^{-2} moles}{1 Liter}](/tpl/images/0680/9750/734fa.png) =2.09*10⁻²
=2.09*10⁻² 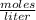
![[I_{2} ]=\frac{4.14*10^{-2} moles}{1 Liter}](/tpl/images/0680/9750/92c8b.png) =4.14*10⁻²
=4.14*10⁻² ![[I_{2} ]=\frac{0.280 moles}{1 Liter}](/tpl/images/0680/9750/e5237.png) = 0.280
= 0.280