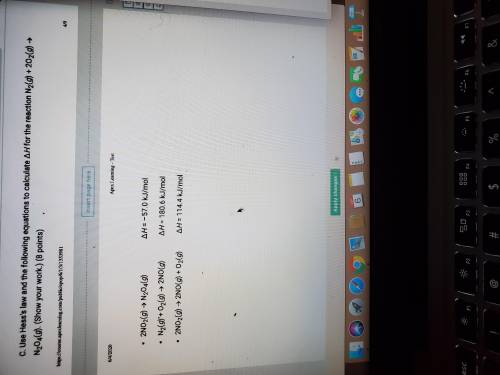
Use Hess law and the following equations to calculate H for the reactio below
...


Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, jabper5522
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1


Chemistry, 22.06.2019 16:00, julesperez22
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3
Do you know the correct answer?
Questions in other subjects:




Health, 13.10.2020 02:01




Mathematics, 13.10.2020 02:01


Mathematics, 13.10.2020 02:01