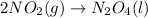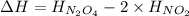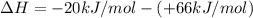Chemistry, 07.06.2020 13:57, Spence8900
PLZ HELP For the reaction: 2NO2(g) → N2O4(l),
the ΔH of the reactants, two moles of NO2 (g), is + 66 kJ/mol,
and the ΔH of the products, N2O4 (l), is -20 kJ/mol.
Which of the following shows the ΔH (change in enthalpy) for the reaction as a whole?
Question 8 options:
ΔrxnH =(- 20 kJ/mol) / (+66 kJ/mol)
ΔrxnH = (+66 kJ/mol) + (- 20 kJ/mol)
ΔrxnH =(-20 kJ/mol) - (+ 66 kJ/mol)
ΔrxnH =(+66 kJ/mol) - (- 20 kJ/mol)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:40, kellypechacekoyc1b3
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 10:30, freddhendrickss
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2

Chemistry, 22.06.2019 13:00, netflixacc0107
Amixture with the same composition throughout is!
Answers: 1

Chemistry, 23.06.2019 00:00, jasmin5285
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
Do you know the correct answer?
PLZ HELP For the reaction: 2NO2(g) → N2O4(l),
the ΔH of the reactants, two moles of NO2 (g), is + 6...
Questions in other subjects:





Mathematics, 10.06.2020 06:57

Physics, 10.06.2020 06:57

Mathematics, 10.06.2020 06:57

Physics, 10.06.2020 06:57


Mathematics, 10.06.2020 06:57


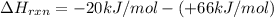
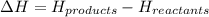
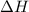 = enthalpy change = ?
= enthalpy change = ?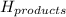 = enthalpy of products
= enthalpy of products 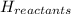 = enthalpy of reactants
= enthalpy of reactants