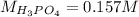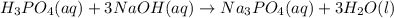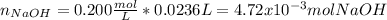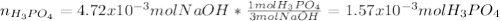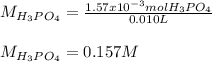Chemistry, 07.06.2020 04:57, isaiahst573
f 23.6 mL of 0.200 M NaOH is required to neutralize 10.00 mL of a H3PO4 solution , what is the concentration of the phosphoric acid solution?Start by balancing the equation for the reaction: H3PO4(aq) + NaOH(aq) → Na3PO4(aq) + H2O(l)

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 23:00, maddyleighanne
Arectangle has a diagonal 20 inches long that forms angles of 60 and 30 with the sides. find the perimeter of the rectangle. for geometry
Answers: 3

Chemistry, 23.06.2019 05:40, shelbylynn1093
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2

Chemistry, 23.06.2019 05:50, kawaunmartinjr10
Aseismic wave is energy released as the result of rock movement along a fault. t or f ?
Answers: 1

Chemistry, 23.06.2019 06:00, hopechinn6646
Complete the sentences to best explain the ranking. match the words below to the appropriate blanks in the sentences. a less polar bondhigher molar massion-dipole forcesstronger intermolecular forcesdipole-dipole forcesdispersion forceshydrogen bonding1. h2s and h2se exhibit the following intermolecular forces:.2. therefore, when comparing h2s and h2se the one with a has a higher boiling point .3. the strongest intermolecular force exhibited by h2o is . therefore, when comparing h2se and h2o the one with has a higher boiling point.
Answers: 1
Do you know the correct answer?
f 23.6 mL of 0.200 M NaOH is required to neutralize 10.00 mL of a H3PO4 solution , what is the conce...
Questions in other subjects:

Mathematics, 18.03.2021 20:00





Mathematics, 18.03.2021 20:00

Geography, 18.03.2021 20:00