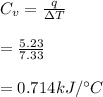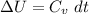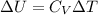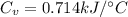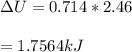Chemistry, 07.06.2020 03:58, Pookaapoo8832
Next, a chemical reaction of interest was conducted in the same constant volume calorimeter. The neutralization reaction of HCl(aq) with NaOH(aq) caused the temperature of the calorimeter to rise by 2.46 °C. What is the change in internal energy ΔU of the neutralization reaction in kJ?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 19:30, gracieisweird12
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3


Chemistry, 23.06.2019 04:31, cassiuspricerules
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2
Do you know the correct answer?
Next, a chemical reaction of interest was conducted in the same constant volume calorimeter. The neu...
Questions in other subjects:






History, 29.06.2019 08:00




Mathematics, 29.06.2019 08:00