
Chemistry, 07.06.2020 00:03, dylalove4963
Given the diagram representing a closed system at constant temperature: Which statement describes this system at equilibrium? 1. The mass of H 2 O( ) equals the mass of H 2 O (g). 2. The volume of H 2 O ( ) equals the volume of H 2 O (g). 3. The number of moles of H2 O ( ) equals the number of moles of H2 O (g). 4. The rate of evaporation of H2 O ( ) equals the rate of condensation of H2 O (g).
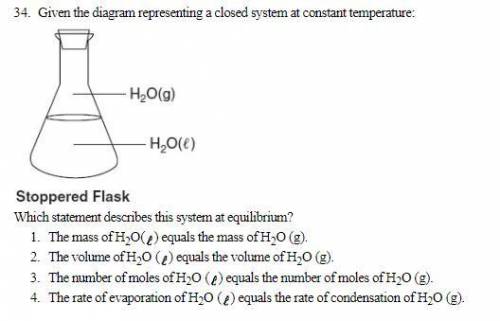

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, myamiller558
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 10:00, emfranco1
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 11:20, Jessicadiaz8602
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1
Do you know the correct answer?
Given the diagram representing a closed system at constant temperature: Which statement describes th...
Questions in other subjects:

Mathematics, 20.10.2020 07:01

Mathematics, 20.10.2020 07:01





Mathematics, 20.10.2020 07:01

Mathematics, 20.10.2020 07:01

Mathematics, 20.10.2020 07:01

Mathematics, 20.10.2020 07:01






