Chemistry, 16.12.2019 23:31, iliketurtures
Copper metal (cu) reacts with silver nitrate (agno3) in aqueous solution to form ag and cu(no3)2. an excess of agno3 is present. the balanced chemical equation is shown below.
cu + 2agno3 > cu(no3)2 + 2ag
the molar mass of cu is 63.5 g/mol. the molar mass of ag is 107.9 g/mol. what mass, in grams, of ag is produced from reaction of 31.75 g of cu?
26.95
107.9
215.91
431.82

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, ksalinas7404
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 00:30, megaaan214p61pb7
Which compounds have the empirical formula ch2o? a. c2h4o2 b. c3h6o3 c. ch2o2 d. c5h10o5 e. c6h12o6
Answers: 3

Chemistry, 22.06.2019 14:00, BrandyLeach01
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3
Do you know the correct answer?
Copper metal (cu) reacts with silver nitrate (agno3) in aqueous solution to form ag and cu(no3)2. an...
Questions in other subjects:




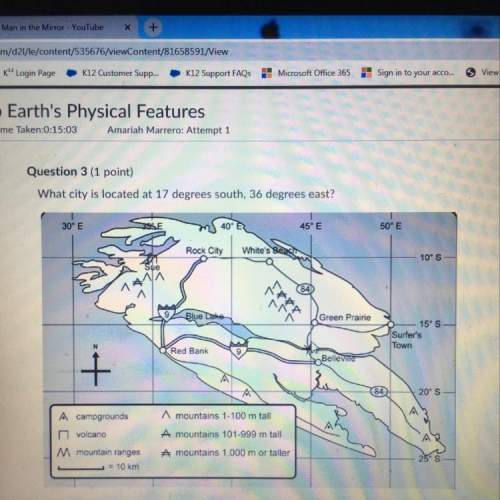
Business, 25.09.2021 14:00


Mathematics, 25.09.2021 14:00



Mathematics, 25.09.2021 14:00

Mathematics, 25.09.2021 14:00