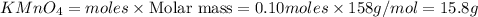Chemistry, 06.06.2020 05:01, kestegag7162
2KMnO4= K2MnO4+ MnO2+O2 how many grams of KMnO4 are required to produce 1.60 grams of O2

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:30, cynthiagutierrez65
Given that the molar mass of nano3 is 85.00 g/mol, what mass of nano3 is needed to make 4.50 l of a 1.50 m nano3solution? use .6.75 g18.9 g255 g574 g
Answers: 1


Chemistry, 22.06.2019 09:00, dante766
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 16:50, mathiscool7
Ajet plane is speeding down the runway during takeoff. air resistance is not negligible. identify the forces on the jet.
Answers: 3
Do you know the correct answer?
2KMnO4= K2MnO4+ MnO2+O2 how many grams of KMnO4 are required to produce 1.60 grams of O2...
Questions in other subjects:

Health, 16.04.2020 19:36

Computers and Technology, 16.04.2020 19:36

Mathematics, 16.04.2020 19:36


English, 16.04.2020 19:36

Mathematics, 16.04.2020 19:36




English, 16.04.2020 19:36


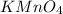 will be required to produce 1.60 grams of
will be required to produce 1.60 grams of 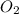
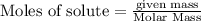
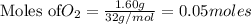
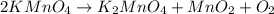
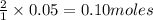 of
of