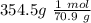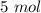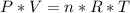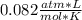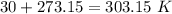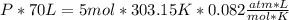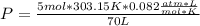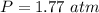Chemistry, 06.06.2020 04:01, khenalilovespandas
354.5 g of chlorine gas (MW = 70.9 g/mol) is held in a vessel with a fixed volume of 70. L.
What is the pressure of the gas in atmospheres if it's temperature is 30.0°C?
___ atm

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 05:00, mprjug6
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 4.20 mol fe and 6.70 mol nio(oh) react?
Answers: 3

Chemistry, 23.06.2019 05:30, zeesharpe05
If c + di is a point on the circle, then | c + di |=
Answers: 2
Do you know the correct answer?
354.5 g of chlorine gas (MW = 70.9 g/mol) is held in a vessel with a fixed volume of 70. L.
What is...
Questions in other subjects:



Biology, 23.06.2019 07:00


Geography, 23.06.2019 07:00

Biology, 23.06.2019 07:00



Mathematics, 23.06.2019 07:00