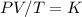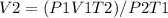Chemistry, 05.06.2020 19:04, ashl3yisbored
A gas has a volume of 1140 ml at 37 ºC and 82.6 kPa pressure. Calculate its volume at STP.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, jadepotts3965
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2

Chemistry, 22.06.2019 09:30, strevino9178
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1


Chemistry, 22.06.2019 12:30, nekathadon
The bond energy for the van der waals bond between two helium atoms is 7.9×10−4ev. assuming that the average kinetic energy of a helium atom is (3/2)kbt, at what temperature is the average kinetic energy equal to the bond energy between two helium atoms
Answers: 1
Do you know the correct answer?
A gas has a volume of 1140 ml at 37 ºC and 82.6 kPa pressure. Calculate its volume at STP....
Questions in other subjects:

English, 25.03.2021 19:50

Mathematics, 25.03.2021 19:50




English, 25.03.2021 19:50


Mathematics, 25.03.2021 19:50


Geography, 25.03.2021 19:50