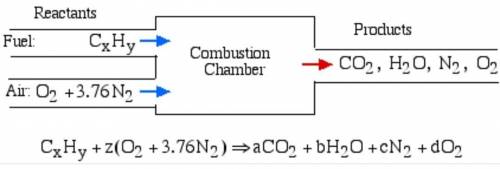
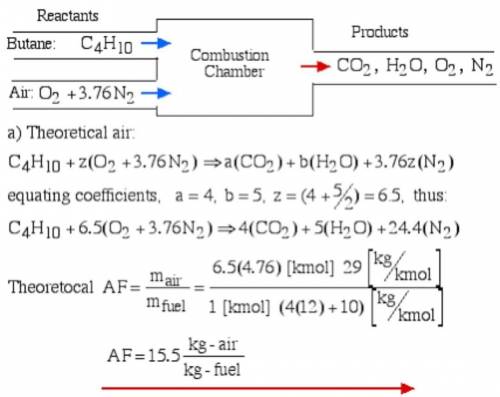
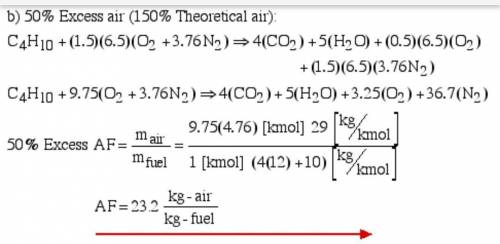
6.) A hydrocarbon molecule of
formula CxHy is completely
burnt in excess oxygen. The
comb...


Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, jojomgarcia01
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2


Chemistry, 23.06.2019 07:00, jstyopin
In order for a high temperature boiler or steam engine to produce superheated water, or steam: the heat source must be greater than 100°c the water must be permitted to evaporate quickly the system must be sealed and become pressurized above atmospheric pressure the vapor pressure must be kept below 760 mm(hg)
Answers: 1

Chemistry, 23.06.2019 07:00, tiarafaimealelei
Select the correct answer. why are scientific models important in the study of science? a. they always involve critical mathematical calculations. b. they scientists understand complex ideas and objects that aren’t easy to handle. c. they enable scientists to popularize their work in society. d. they are required when conducting any peer review process. e. they are necessary for turning a hypothesis into a law.
Answers: 2
Do you know the correct answer?
Questions in other subjects:


Computers and Technology, 01.11.2019 00:31

Biology, 01.11.2019 00:31



Chemistry, 01.11.2019 00:31

Chemistry, 01.11.2019 00:31