HELP PLEASE!!
1.
Above is a solubility curve for KNO3
Solubility has nothi...

HELP PLEASE!!
1.
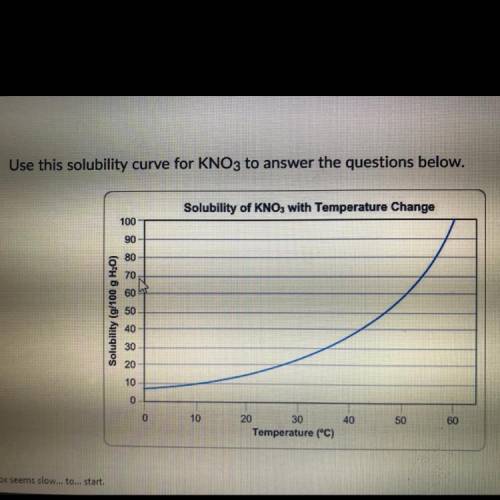
Above is a solubility curve for KNO3
Solubility has nothing to do with the speed of dissolving; it’s a measure of how much salt will dissolve at given temperature.
The y-axis of the graph shows you how much KNO3 will dissolve in 100 g of water. In other words, it tells you the maximum amount of solute that will dissolve at different temperatures.
The x-axis tells you the minimum temperature needed to dissolve different amounts if KNO3 in 100g of water.
Approximately how many grams of KNO3 will dissolve in 100g of water at 0 degrees Celsius?
Type in the number only; no units. Round your answer to the nearest whole number.
2.
Approximately what is the minimum temperature needed to dissolve 10g of KNO3 in 100G of H20?
Type in the number only; no units. Round your answer to the nearest whole number.
3.
Imagine that you have 100g of water.
You start dissolving KNO3 in the water and you find that after you’ve dissolved about 55g of KNO3 you can’t dissolve any more; it just sinks to the bottom.
Approximately what is the temperature of water?
Type in the number only; no units. Round your answer to the nearest whole number.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:30, ruleolivas
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3


Chemistry, 22.06.2019 11:40, tatemelliott
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3
Do you know the correct answer?
Questions in other subjects:

Mathematics, 02.02.2021 14:00



English, 02.02.2021 14:00


Arts, 02.02.2021 14:00

World Languages, 02.02.2021 14:00


Biology, 02.02.2021 14:00

Chemistry, 02.02.2021 14:00






