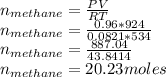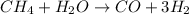Chemistry, 04.06.2020 13:30, zaylencollins55
The reform reaction between steam and gaseous methane () produces "synthesis gas," a mixture of carbon monoxide gas and dihydrogen gas. Synthesis gas is one of the most widely used industrial chemicals, and is the major industrial source of hydrogen. Suppose a chemical engineer studying a new catalyst for the reform reaction finds that liters per second of methane are consumed when the reaction is run at and . Calculate the r

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, jadepotts3965
Calculate the change in entropy if br2(l) is converted into gaseous br atoms. s° for br2(l) = 152.2 j/(mol•k) s° for br2(g) = 245.5 j/(mol•k) s° for br(g) = 175.0 j/(mol•k)
Answers: 2


Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Do you know the correct answer?
The reform reaction between steam and gaseous methane () produces "synthesis gas," a mixture of carb...
Questions in other subjects:



Mathematics, 28.04.2021 22:00

Mathematics, 28.04.2021 22:00


Mathematics, 28.04.2021 22:00



Mathematics, 28.04.2021 22:00

Computers and Technology, 28.04.2021 22:00