The tabulated data were collected for this reaction:
CH3Cl(g) + 3Cl2(g) → CCl4(g) + 3HCl(g)
<...

Chemistry, 04.06.2020 13:28, zahinparvez69
The tabulated data were collected for this reaction:
CH3Cl(g) + 3Cl2(g) → CCl4(g) + 3HCl(g)
[CH3Cl] (M) [Cl2] (M) Initial Rate (M/s)
0.050 0.050 0.014
0.100 0.050 0.029
0.100 0.100 0.041
0.200 0.200 0.115
(a) Write an expression for the reaction rate law and calculate the value of the rate constant, k.
(b) What is the overall order of the reaction?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, aylengarcia090
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 16:30, Eddie997
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2


Chemistry, 23.06.2019 05:40, shelbylynn1093
Why is it incorrect to balance a chemical equation by changing the subscripts? explain.
Answers: 2
Do you know the correct answer?
Questions in other subjects:



History, 12.08.2021 21:40

Mathematics, 12.08.2021 21:40

English, 12.08.2021 21:40


Physics, 12.08.2021 21:40


Mathematics, 12.08.2021 21:40

English, 12.08.2021 21:40


![Rate = k [CH_3 Cl] [Cl_2]^{0.5}](/tpl/images/0675/8688/f7f7b.png)
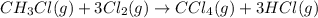
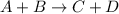 , the rate of backward reaction is given by:
, the rate of backward reaction is given by: ![Rate = k [A]^{a} [B]^{b}\\k = \frac{Rate}{ [A]^{a} [B]^{b}}\\k = \frac{Rate}{ [CH_3 Cl]^{a} [Cl_2]^{b}}](/tpl/images/0675/8688/ebbf5.png)
![0.014 / [0.05]^a [0.05]^b = 00.029/ [0.100]^a [0.05]^b\\0.014 / [0.05]^a [0.05]^b = 00.029/ [2*0.05]^a [0.05]^b\\0.014 / = 0.029/ 2^a\\2^a = 2.07\\a = 1](/tpl/images/0675/8688/247cc.png)
![0.041 / [0.100]^a [0.100]^b = 0.115 / [0.200]^a [0.200]^b\\0.041 / [0.100]^a [0.100]^b = 0.115 / [2 * 0.100]^a [2 * 0.100]^b\\](/tpl/images/0675/8688/0c6b5.png)
![0.041 = 0.115 / [2 ]^a [2]^b\\ \[[2 ]^a [2]^b = 0.115/0.041\\ \[[2 ]^a [2]^b = 2.80\\\[[2 ]^1 [2]^b = 2.80\\\[[2]^b = 1.40\\b = \frac{ln 1.4}{ln 2} \\b = 0.5](/tpl/images/0675/8688/ae2b8.png)
![k = \frac{Rate}{ [CH_3 Cl]^{a} [Cl_2]^{b}}](/tpl/images/0675/8688/c4a73.png) then becomes:
then becomes:![k = 0.014 / ( [0.050] [0.050]^(0.5) )\\k = 1.25](/tpl/images/0675/8688/5313f.png)




