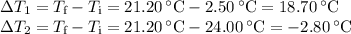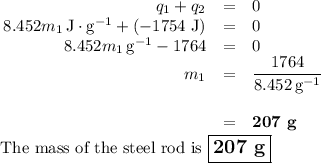CHEM EXPERT NEEDED***
A volume of 150.0 mL of H2O is initially at 24.00 °C. A chilled steel rod at 2.50 °C is placed in the water and the final temperature of the system is 21.20 °C.
Specific heat of water = 4.184 J/(g⋅∘C) and the specific heat of steel = 0.452 J/(g⋅∘C)
Write the equation and calculate the mass of the rod.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, raizagisselle1694
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3


Chemistry, 22.06.2019 12:00, KKHeffner02
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1
Do you know the correct answer?
CHEM EXPERT NEEDED***
A volume of 150.0 mL of H2O is initially at 24.00 °C. A chilled steel rod at...
Questions in other subjects:

History, 13.10.2020 05:01


Mathematics, 13.10.2020 05:01




Mathematics, 13.10.2020 05:01

Chemistry, 13.10.2020 05:01


Chemistry, 13.10.2020 05:01