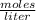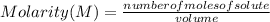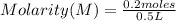Chemistry, 04.06.2020 13:16, Brandon4188
Calculate the molarity of a solution containing 0.2 mol of sodium hydroxide dissolved in 0.5 L of water. Be sure to report your answer in proper significant figures and use the appropriate symbol.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:30, leilanimontes714
Asample of silver (with work function ? = 4.52 ev) is exposed to an ultraviolet light source (? = 200 nm), which results in the ejection of photoelectrons. what changes will be observed if: silver is replaced with copper (? = 5.10 ev) more photoelectrons ejected no photoelectrons are emitted fewer photoelectrons ejected more energetic photoelectrons (on average) less energetic photoelectrons (on average)
Answers: 3

Chemistry, 21.06.2019 22:30, erinxmeow8
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons, neutrons, electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2


Chemistry, 22.06.2019 03:50, AysiaRamosLee
What is the temperature of one mole of helium gas at stp?
Answers: 3
Do you know the correct answer?
Calculate the molarity of a solution containing 0.2 mol of sodium hydroxide dissolved in 0.5 L of wa...
Questions in other subjects:

Chemistry, 14.02.2021 02:30

Mathematics, 14.02.2021 02:30

History, 14.02.2021 02:30



Geography, 14.02.2021 02:30

Mathematics, 14.02.2021 02:30

Computers and Technology, 14.02.2021 02:30


Mathematics, 14.02.2021 02:30