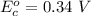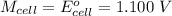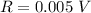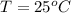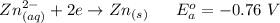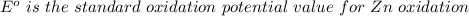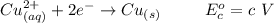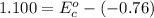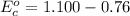Chemistry, 04.06.2020 13:20, saggin2454
Using the cell voltage measured for the first cell studied, with cell chemistry Zn/Zn^2+ \ Cu^2+/Cu, and the known half life potential for Zn^2/Zn calculate the reduction potential for Cu^2+/Cu and enter value below.
The information received for this problem were the values obtained during an online lab:
Cu xM cell voltage:1.100 V
Range: 0.005 V
Temp: 25 degrees C

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 08:30, lowkstahyna
What percentage of energy used in the u. s is produced from fossil fuels
Answers: 2
Do you know the correct answer?
Using the cell voltage measured for the first cell studied, with cell chemistry Zn/Zn^2+ \ Cu^2+/Cu,...
Questions in other subjects:


Chemistry, 09.12.2020 19:00

Chemistry, 09.12.2020 19:00


Chemistry, 09.12.2020 19:00

Mathematics, 09.12.2020 19:00


Mathematics, 09.12.2020 19:00



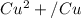 is
is