
Chemistry, 03.06.2020 11:57, tylermdons
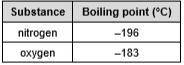
Air is a mixture of nitrogen, oxygen and smaller amounts of many other substances. If air is filtered and cooled to low temperatures it becomes a mixture of liquids. Nitrogen gas and liquid oxygen can be separated from this ‘liquid air’ by fractional distillation. The table shows the boiling points of nitrogen and oxygen. Use your knowledge and understanding of fractional distillation, and the information given, to explain how nitrogen and oxygen can be separated from air.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, officialgraciela67
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1


Chemistry, 22.06.2019 17:00, smelcher3900
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 23.06.2019 01:00, Angelofpink1143
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
Do you know the correct answer?
Air is a mixture of nitrogen, oxygen and smaller amounts of many other substances. If air is filtere...
Questions in other subjects:

SAT, 09.02.2022 02:10

Mathematics, 09.02.2022 02:10

SAT, 09.02.2022 02:10


Mathematics, 09.02.2022 02:10


Mathematics, 09.02.2022 02:10








