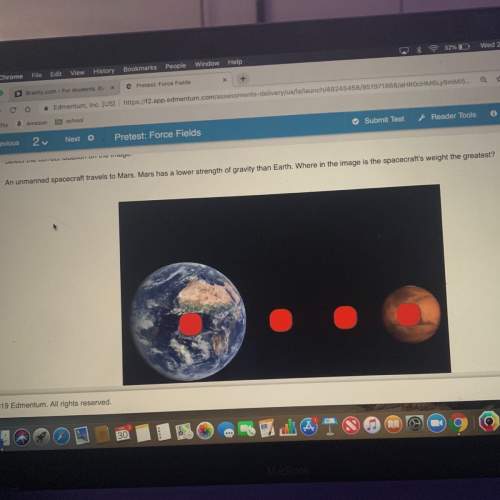
Chemistry, 02.06.2020 04:59, harleygirl9451
How much heat is required to vaporize 166g of water at it's boiling point, if water's heat of vaporization is equal to 40.7kJ/mol?
kJ

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, tgraveslaylay2743
Bose-einstein condensation occurs at what temperature?
Answers: 2

Chemistry, 21.06.2019 22:00, applereams
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 21.06.2019 23:30, ashleyjaslin
Calculate the expected ph values of the buffer systems from the experiments (a, b,c, d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2
Do you know the correct answer?
How much heat is required to vaporize 166g of water at it's boiling point, if water's heat of vapori...
Questions in other subjects:


Mathematics, 20.07.2019 20:00

Mathematics, 20.07.2019 20:00

Biology, 20.07.2019 20:00

Mathematics, 20.07.2019 20:00

Mathematics, 20.07.2019 20:00


History, 20.07.2019 20:00

History, 20.07.2019 20:00