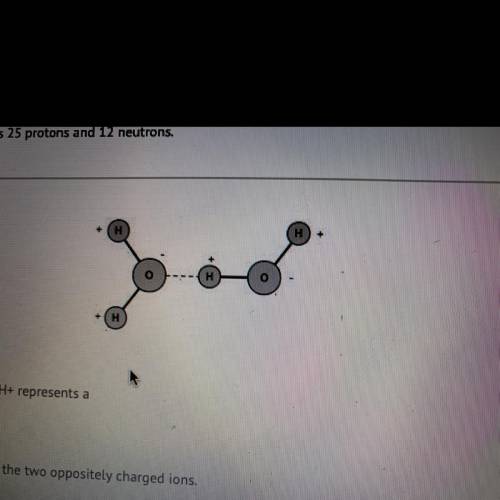
The dotted line between 0- and H+ represents a
A)
covalent bond between the two opposite...

The dotted line between 0- and H+ represents a
A)
covalent bond between the two oppositely charged ions.
B)
hydrogen bond, a weak bond between a hydrogen and an electronegative
atom.
C)
strong, short-distance bond that is responsible for the unique properties of
water.
polar covalent bond that results from the unequal and opposite charges of
the ions.
D)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, johnsont8377
Avariable that is not being directly tested during an experiment should be changed varied experimented controlled
Answers: 1

Chemistry, 22.06.2019 17:30, kiaramccurty
What type of organic molecule comprises the majority of a potato?
Answers: 1

Chemistry, 22.06.2019 17:30, latezwardjr15
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 18:40, bananaslada
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1
Do you know the correct answer?
Questions in other subjects:

History, 29.01.2021 01:00

History, 29.01.2021 01:00


History, 29.01.2021 01:00

Social Studies, 29.01.2021 01:00

Mathematics, 29.01.2021 01:00




Mathematics, 29.01.2021 01:00






