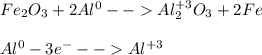Given the balanced equation representing a reaction:
Fe2O3 + 2Al Al2O3 + 2Fe
...

Chemistry, 01.06.2020 18:57, shayleewright
Given the balanced equation representing a reaction:
Fe2O3 + 2Al Al2O3 + 2Fe
During this reaction, the oxidation number of Al changes from
A) +3 to 0 as electrons are transferred
B) +3 to 0 as protons are transferred
C) 0 to +3 as electrons are transferred
D) 0 to +3 as protons are transferred

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:10, lilyjordan5972
How do forces between particles in gases compare to forces in the other states of matter? o a. the forces in gases are stronger than forces in solids but weaker than forces in liquids. o b. the forces in gases are weaker than forces in solids but stronger than forces in liquids. o c. the forces in gases are weaker than forces in solids and liquids. o d. the forces in gases are stronger than forces in solids and liquids. submit
Answers: 1

Chemistry, 22.06.2019 05:30, jzjajsbdb8035
Which other elements contain the same number of outer electrons as sodium
Answers: 3

Chemistry, 22.06.2019 09:00, oliviacolaizzi
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 14:00, coylenoah0
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
Do you know the correct answer?
Questions in other subjects:





History, 22.04.2020 00:17

Mathematics, 22.04.2020 00:17

Mathematics, 22.04.2020 00:17

Biology, 22.04.2020 00:17