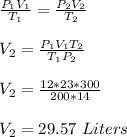Chemistry, 01.06.2020 12:57, potato3999
If I initially have a gas at a pressure of 12 atm, a volume of 23 Liters and a temperature of 200K, and then I raise the pressure to 14 atm and increase the temperature to 300K, what is the new volume?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 03:00, Cheyenne7327
Analyze the reaction to determine whether the reaction is exothermic or endothermic. explain your reasoning.
Answers: 1


Chemistry, 23.06.2019 11:50, halllawson
What is the oxidation half-reaction for this unbalanced redox equation? cr2o72– + fe2+ → cr3+ + fe3+ cr3+ → cr2o72– cr2o72– → cr3+ fe3+ → fe2+ fe2+ → fe3+?
Answers: 2

Chemistry, 23.06.2019 15:00, ctyrector
20 look at the clock and the data table below. based on the data and on your knowledge of potential and kinetic energy, what is the best conclusion you can make about potential and kinetic energy? the total amount of energy stays the same. the clock has the most potential energy at point b since it is moving the fastest. there is always more potential energy than kinetic energy. potential energy can never be 0, but you can have 0 kinetic energy.
Answers: 1
Do you know the correct answer?
If I initially have a gas at a pressure of 12 atm, a volume of 23 Liters and a temperature of 200K,...
Questions in other subjects:


Arts, 20.08.2020 01:01


English, 20.08.2020 01:01

Mathematics, 20.08.2020 01:01

Mathematics, 20.08.2020 01:01



Social Studies, 20.08.2020 01:01