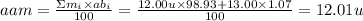Chemistry, 31.05.2020 06:58, ambercuevas2707
Attempt 1
The element carbon has two naturally occurring isotopes. The isotopic masses and abundances of these isotopes are shown
in the table.
Isotope
12c
13C
Isotopic mass (u)
12.00
Abundance (%)
98.93
13.00
1.07
Calculate the average atomic mass of carbon to two digits after the decimal point.
average atomic mass =

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, Dkhaurithompson
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1



Chemistry, 23.06.2019 07:50, alexusnicole817
Asolution is produced in which water is the solvent and there are four solutes. which of the solutes can dissolve better if the solution is heated?
Answers: 1
Do you know the correct answer?
Attempt 1
The element carbon has two naturally occurring isotopes. The isotopic masses and abu...
The element carbon has two naturally occurring isotopes. The isotopic masses and abu...
Questions in other subjects:










History, 16.01.2020 21:31


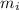 ) and the abundance of each isotope (
) and the abundance of each isotope (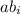 ) using the following expression.
) using the following expression.