
Question 17 In the Haber reaction, patented by German chemist Fritz Haber in 1908, dinitrogen gas combines with dihydrogen gas to produce gaseous ammonia. This reaction is now the first step taken to make most of the world's fertilizer. Suppose a chemical engineer studying a new catalyst for the Haber reaction finds that 786. liters per second of dinitrogen are consumed when the reaction is run at 222.°C and 0.35atm. Calculate the rate at which ammonia is being produced. Give your answer in kilograms per second. Round your answer to 2 significant digits.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2



Chemistry, 23.06.2019 03:00, sharondacarruth1656
Is it safe to take 450mg of diphenhydramine hydrochloride?
Answers: 1
Do you know the correct answer?
Question 17 In the Haber reaction, patented by German chemist Fritz Haber in 1908, dinitrogen gas co...
Questions in other subjects:





Mathematics, 06.10.2019 04:00




Mathematics, 06.10.2019 04:00

Business, 06.10.2019 04:00


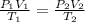
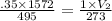
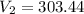 liter .
liter . 



