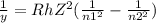An electron in an unknown energy level of a hydrogen atom transitions to the n=2 level and emits a photon with wavelength 410 nm in the process. What was the initial energy level? Use R[infinity]=2.179×10−18J for the hydrogen atom Rydberg constant. Use h=6.626×10−34 Js for Planck's constant. Use c=2.998×108ms for the speed of light. Your answer should be a whole number.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 00:30, danielmartinez024m
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1

Do you know the correct answer?
An electron in an unknown energy level of a hydrogen atom transitions to the n=2 level and emits a p...
Questions in other subjects:

English, 16.05.2020 19:57

Mathematics, 16.05.2020 19:57





Mathematics, 16.05.2020 19:57

Spanish, 16.05.2020 19:57


Mathematics, 16.05.2020 19:57