
Chemistry, 31.05.2020 03:00, cookiemonster0476
Hard water often contains dissolved Ca2+ and Mg2+ ions. One way to soften water is to add phosphates. The phosphate ion forms insoluble precipitates with calcium and magnesium ions, removing them from solution. Suppose that a solution is 0.054 M in calcium chloride and 0.093 M in magnesium nitrate. What mass of sodium phosphate would have to be added to 1.4 L of this solution to completely eliminate the hard water ions? Assume complete reaction. Enter a numerical answer only, in terms of grams to two significant figures.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 23.06.2019 05:40, MyChannelBruh6896
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1
Do you know the correct answer?
Hard water often contains dissolved Ca2+ and Mg2+ ions. One way to soften water is to add phosphates...
Questions in other subjects:


Mathematics, 30.10.2020 03:20

Biology, 30.10.2020 03:20

Mathematics, 30.10.2020 03:20

Mathematics, 30.10.2020 03:20

Mathematics, 30.10.2020 03:20


English, 30.10.2020 03:20

Mathematics, 30.10.2020 03:20


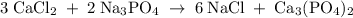
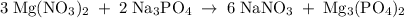
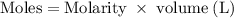
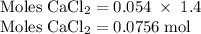
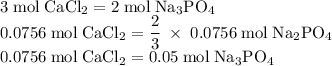
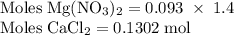
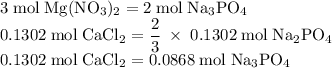
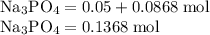
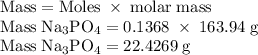
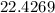 grams of sodium phosphate must be added to 1.4 L of this solution to completely eliminate the hard water ions
grams of sodium phosphate must be added to 1.4 L of this solution to completely eliminate the hard water ions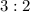
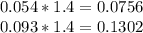
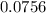 mole of CaCl2 is equal to
mole of CaCl2 is equal to 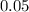 Na3PO4
Na3PO4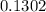 mole of CaCl2 is equal to
mole of CaCl2 is equal to 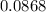 Na3PO4
Na3PO4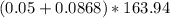 g/mol
g/mol 



