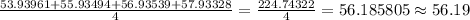A certain element X has four isotopes. 5.845% of X has a mass of 53.93961 amu. 91.75% of X has a mass of 55.93494 amu. 2.123% of X has a mass of 56.93539 amu. 0.2820% of X has a mass of 57.93328 amu. What is the average atomic mass of element X? Express your answer numerically to four significant figures.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, brittanysanders
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 07:20, camillexv2668
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 12:30, robert7248
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 17:30, shookiegriffin
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1
Do you know the correct answer?
A certain element X has four isotopes. 5.845% of X has a mass of 53.93961 amu. 91.75% of X has a mas...
Questions in other subjects:


Physics, 01.10.2021 15:20







Mathematics, 01.10.2021 15:20