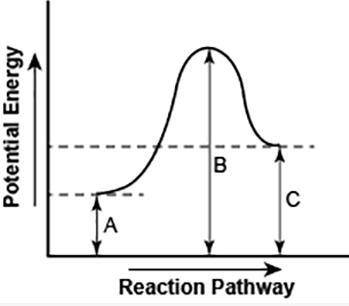
The diagram shows the potential energy changes for a reaction pathway.
Part 1: Does the...

The diagram shows the potential energy changes for a reaction pathway.
Part 1: Does the diagram illustrate an endothermic or an exothermic reaction? Give reasons in support of your answer.
Part 2: Describe how you can determine the total change in enthalpy and activation energy from the diagram and if each is positive or negative.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:04, brandon1748
Two liquids are shaken together in a test tube to produce a mixture that quickly separates into two layers. which of the following best describes the behavior of the above pair of substances? soluble insoluble miscible immiscible
Answers: 1

Chemistry, 22.06.2019 00:20, paulawells11
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 23.06.2019 01:00, birdman2540
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
Do you know the correct answer?
Questions in other subjects:


Mathematics, 30.10.2020 21:50


Mathematics, 30.10.2020 21:50

Health, 30.10.2020 21:50










