
Chemistry, 29.05.2020 22:58, yulimariu27
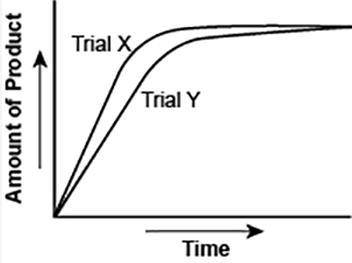
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different concentration of reactant was used during each trial, whereas the other factors were kept constant.
Which of the following statements explains which trial has a lower concentration of the reactant?
A) Trial X, because the final volume of product formed is lower than Trial Y.
B) Trial X, because this reaction was initially fast and later stopped completely.
C) Trial Y, because the reaction was initially slow and later stopped completely.
D) Trial Y, because the volume of product formed per unit time is lower than Trial X.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 17:00, alevans7144
Why do sodium and neon have vastly different chemical and physical properties despite having similar atomic masses?
Answers: 2

Chemistry, 21.06.2019 21:30, gatorr2010
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 05:30, alaynagrace1111
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 23:30, Xavier8247
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3
Do you know the correct answer?
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different...
Questions in other subjects:

Mathematics, 25.03.2020 20:30




Mathematics, 25.03.2020 20:30

History, 25.03.2020 20:30


Mathematics, 25.03.2020 20:30

Spanish, 25.03.2020 20:30






