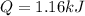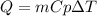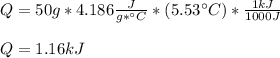Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:10, mistiehaas
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2



Chemistry, 23.06.2019 05:00, pmbeachy3102
If 15 drops of ethanol from a medicine dropper weigh 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? density of ethanol is ethanol is 0.80g/ml.
Answers: 2
Do you know the correct answer?
How much heat, in kJ, is required to raise the temperature of 50 g of water by 5.53°C? (Round to the...
Questions in other subjects:

Mathematics, 13.01.2020 20:31

Biology, 13.01.2020 20:31


Mathematics, 13.01.2020 20:31

Geography, 13.01.2020 20:31

Physics, 13.01.2020 20:31

Mathematics, 13.01.2020 20:31

Biology, 13.01.2020 20:31

Mathematics, 13.01.2020 20:31

Chemistry, 13.01.2020 20:31