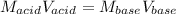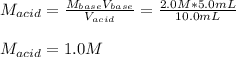Chemistry, 29.05.2020 20:01, nenelacayo07
In a titration, 5.0 ml of a 2.0 M NaOH (aq) solutions exactly neutralizes 10.0 ml of an HCL (aq) solution. what is the concentration of the HCl (aq) solution?

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 05:30, jalynholden07
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3

Chemistry, 23.06.2019 14:00, kealinwiley
Which of the following is not a result when a change to an equilibrium system is applied? (2 points) increasing the rate of the forward reaction will cause a shift to the left. increasing the rate of the reverse reaction will cause a shift to the left. decreasing the rate of the forward reaction will cause a shift to the left. decreasing the rate of the reverse reaction will cause a shift to the right.
Answers: 1
Do you know the correct answer?
In a titration, 5.0 ml of a 2.0 M NaOH (aq) solutions exactly neutralizes 10.0 ml of an HCL (aq) sol...
Questions in other subjects:







Engineering, 11.07.2019 03:20


Engineering, 11.07.2019 03:20