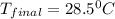Chemistry, 29.05.2020 20:00, rawaanasser12245
The specific heat of mercury is 0.138 J/g Co . If 452g of mercury at 85.0 Co are placed in 145g of water at 23.0 Co , what will be the final temperature for both the mercury and the water?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, alexusnicole817
Which of the following signs of a chemical reaction are observed in the reaction of potassium with water? precipitate formed temperature change smell produced gas produced color change
Answers: 2



Chemistry, 23.06.2019 02:30, hailee232
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1
Do you know the correct answer?
The specific heat of mercury is 0.138 J/g Co . If 452g of mercury at 85.0 Co are placed in 145g of w...
Questions in other subjects:


Biology, 28.01.2020 08:31




Mathematics, 28.01.2020 08:31

Social Studies, 28.01.2020 08:31

Physics, 28.01.2020 08:31


Biology, 28.01.2020 08:31


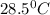
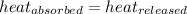
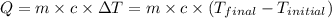
![m_1\times c_1\times (T_{final}-T_1)=-[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/0670/5209/09236.png) .................(1)
.................(1)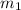 = mass of mercury = 425 g
= mass of mercury = 425 g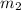 = mass of water = 145 g
= mass of water = 145 g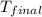 = final temperature = ?
= final temperature = ?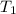 = temperature of mercury =
= temperature of mercury = 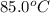
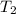 = temperature of water =
= temperature of water = 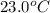
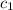 = specific heat of mercury =
= specific heat of mercury = 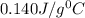
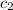 = specific heat of water=
= specific heat of water= 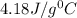
![-425\times 0.140\times (T_{final}-85.0)=[145\times 4.184\times (T_{final}-23.0)]](/tpl/images/0670/5209/c9617.png)