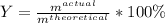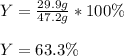In the reaction between CO and Fe3O4, the theoretical yield in an experiment is calculated to be 47.2 g Fe. When a chemistry student carries out the experiment, the actual yield is 29.9 g Fe. Calculate the percentage yield. % Please show all work.
a. .633%
b 157%
c 63.3%
d .157%

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, davidrodriguez122001
Which of the following describes a situation where competition between producers exists
Answers: 1

Chemistry, 23.06.2019 05:40, MyChannelBruh6896
Convert a speed of 201 cm/s to units of inches per minute. also, show the unit analysis by dragging components into the unit‑factor slots.
Answers: 1

Chemistry, 23.06.2019 06:20, Lindsay882
Type the correct answer in each box. balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1

Chemistry, 23.06.2019 06:20, cowboo5000pcl655
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1
Do you know the correct answer?
In the reaction between CO and Fe3O4, the theoretical yield in an experiment is calculated to be 47....
Questions in other subjects:

English, 24.11.2020 01:30

Spanish, 24.11.2020 01:30

Mathematics, 24.11.2020 01:30

Mathematics, 24.11.2020 01:30


World Languages, 24.11.2020 01:30