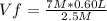I’LL MAKE YOU BRAINLIEST+ FREE POINTS
An industrial-strength pipe cleaner has a molarit...

Chemistry, 29.05.2020 18:58, josephpezza18
I’LL MAKE YOU BRAINLIEST+ FREE POINTS
An industrial-strength pipe cleaner has a molarity (M) of 7.0 M in a 0.60 liter container. To be safely used on the pipes in a home, the
molarity is decreased to 2.5 M. What is the solution's new volume?

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, applejulianamoreno
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 21:30, leenzazou587
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Do you know the correct answer?
Questions in other subjects:

Social Studies, 19.07.2019 02:30

History, 19.07.2019 02:30


History, 19.07.2019 02:30

English, 19.07.2019 02:30



Social Studies, 19.07.2019 02:30

History, 19.07.2019 02:30

Geography, 19.07.2019 02:30