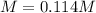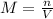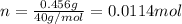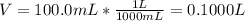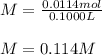Chemistry, 29.05.2020 00:59, tugordochulo1
Si se pesan 0.456 gramos de NaOH y se disuelven en 100.0 ml de solución. ¿cuál es la molaridad?
O 0.114 M.
O 0.134 M
0.001M
1.00 x 10-3 M

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 18:30, lattimorekeonna1
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 19:00, Jasoncookies23
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
Do you know the correct answer?
Si se pesan 0.456 gramos de NaOH y se disuelven en 100.0 ml de solución. ¿cuál es la molaridad?
Questions in other subjects:






Computers and Technology, 08.12.2020 21:50

Social Studies, 08.12.2020 21:50


Mathematics, 08.12.2020 21:50

Chemistry, 08.12.2020 21:50