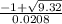Chemistry, 29.05.2020 00:00, putaprincess16
A container has a mixture of NO2 gas and N2O4 gas in equilibrium. The chemical reaction between the two gases is described by the equation 2NO2 ⇌ N2O4. The partial pressure of NO2 is 61.2 MPa and the partial pressure of N2O4 is 38.8 MPa. Several weights are added to the lid of the container, increasing the total pressure on the container to 200 MPa. What are the most likely partial pressures when equilibrium is established again?3.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:40, khan2491
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 12:20, tenleywood
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2
Do you know the correct answer?
A container has a mixture of NO2 gas and N2O4 gas in equilibrium. The chemical reaction between the...
Questions in other subjects:


Mathematics, 12.03.2020 08:20



Mathematics, 12.03.2020 08:21

History, 12.03.2020 08:22



Business, 12.03.2020 08:22


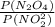
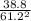
![\frac{200 - P(NO_{2}) }{[P(NO_{2} )]^{2}}](/tpl/images/0669/4562/5f3ab.png)
![[P(NO_{2} )]^{2}](/tpl/images/0669/4562/76f80.png) +
+ 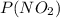 - 200 = 0
- 200 = 0