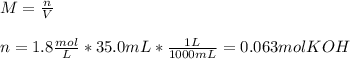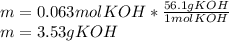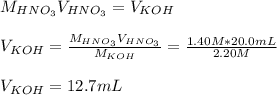Chemistry, 27.05.2020 23:01, sneakersolequeen
PLEASE SHOW WORK
1) You have a solution of acetic acid that has a K a of 3.5 × 10 –8 . If the concentration of the acetic acid were 2.40 M, what would be the concentration of H + at equilibrium?
2) You have a solution that is 1.75 M HCN. If the K a is 9.9 × 10 –8 , calculate the pH of the solution.
3) How many grams of KOH are needed to neutralize 35.0 mL of 1.8 M HCl?
4) How many mL of 2.20 M KOH are needed to neutralize 20.0 mL of 1.40 M HNO 3 ?

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
Do you know the correct answer?
PLEASE SHOW WORK
1) You have a solution of acetic acid that has a K a of 3.5 × 10 –8 . If the...
1) You have a solution of acetic acid that has a K a of 3.5 × 10 –8 . If the...
Questions in other subjects:



Computers and Technology, 10.11.2020 18:30

Chemistry, 10.11.2020 18:30



Spanish, 10.11.2020 18:30


English, 10.11.2020 18:30

Social Studies, 10.11.2020 18:30


![[H^+]_{eq}=0.00029M](/tpl/images/0667/7635/6d441.png)
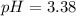
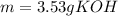
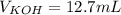
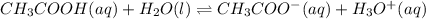
![Ka=\frac{[CH_3COO^-][H_3O^+]}{[CH_3COOH]}](/tpl/images/0667/7635/3cdd5.png)
 due to reaction's extent we have:
due to reaction's extent we have: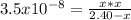
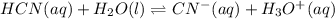
![K=\frac{[CN^-][H_3O^+]}{[HCN]}](/tpl/images/0667/7635/eab5d.png)
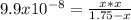
![[H^+]_{eq}=0.000416 M](/tpl/images/0667/7635/3b132.png)
![pH=-log([H^+]_{eq})=-log(0.000416)\\\\pH=3.38](/tpl/images/0667/7635/c9e0e.png)