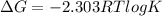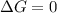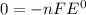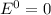Given the chemical reaction: Hg2+(aq) + Cu(s) + Hg(s) + Cu2+(aq)
When the reaction reaches equ...

Chemistry, 28.05.2020 22:04, wolfgirl2431
Given the chemical reaction: Hg2+(aq) + Cu(s) + Hg(s) + Cu2+(aq)
When the reaction reaches equilibrium, the cell potential will be
1
-0.51 V
2.
0.00 V
3.
0.51 V
4.
1.19 V

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, bobbycisar1205
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2


Chemistry, 22.06.2019 14:50, chem1014
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 22.06.2019 18:00, ameliaxbowen7
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 02.10.2019 18:00

History, 02.10.2019 18:00

English, 02.10.2019 18:00




Social Studies, 02.10.2019 18:00


Mathematics, 02.10.2019 18:00