
Chemistry, 28.05.2020 17:58, sofiaarmy12
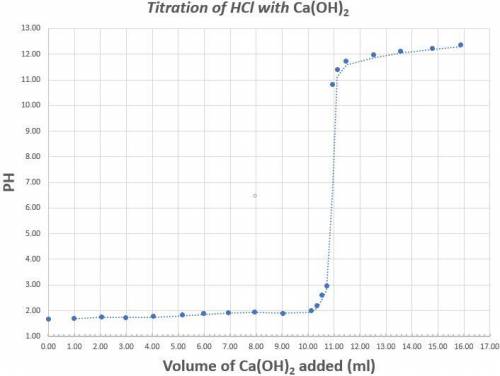
Read the graph to the nearest tenth for both pH and volume of calcium hydroxide. 25.0ml of a 1.70x10-4M solution of Hydrochloric acid was titrated with Calcium hydroxide. The above graph was generated when the Hydrochloric acid was titrated with Calcium hydroxide. Determine the concentration (in M) of the Calcium hydroxide. What is the coefficient of the scientific notation answer for the concentration of Calcium Hydroxide.
Determine the percent error if the known concentration of calcium hydroxide is 6.30x10-4M. (Do not put your answer in scientific notation).

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:00, smartboy2296
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 05:40, Maryjasmine8001
Salicylic acid is a very important acid. it is used to synthesize the aspirin by treating with acetic anhydride. a 0.2015-g sample of salicylic acid was dissolved in a 100.00-ml volumetric flask, and the solution was diluted to the mark. a 10-ml aliquot of this solution was titrated with standard naoh (0.01130 + 0.2% n) to a phenolphthalein faint pink color end point at 19.81 ml. (a) (calculate the normality of the salicylic acid solution used in the titration. (b) assuming the salicylic acid is pure, what is the equivalent weight of the salicylic acid? practice problems for the final exam (continued) (c) (calculate the inherent error in the determination of the equivalent weight you calculated in part (b). use the following absolute errors in the equipment /glassware when calculating the inherent error. 5.00-ml pipet: + 0.02 ml 100-ml volumetric flask: + 0.08 ml analytical balance: + 0.2 mg 25-ml buret: + 0.03 ml
Answers: 2

Chemistry, 22.06.2019 09:10, aleilyg2005
Select the correct answer from each drop-down menu. describe what happens to a carbon-11 atom when it undergoes positron emission. the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3
Do you know the correct answer?
Read the graph to the nearest tenth for both pH and volume of calcium hydroxide. 25.0ml of a 1.70x10...
Questions in other subjects:


Social Studies, 18.12.2020 23:50

History, 18.12.2020 23:50

History, 18.12.2020 23:50

Biology, 18.12.2020 23:50


Mathematics, 18.12.2020 23:50


Mathematics, 18.12.2020 23:50






