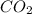Chemistry, 27.05.2020 19:09, alydiale584
A 0.533 g sample of a carbonate salt is added to a flask. The only thing you know is that per formula unit, there is only one carbonate anion, but the identity of the cation (or cations) is unknown. To determine the identity of the cations, 15.0 mL of 1.00 M sulfuric acid is added to the flask. After the resulting reaction completes, excess acid remains. The remaining solution is titrated with 0.5905 M NaOH(aq) and reaches the equivalence point when 29.393 mL of base is added. What is the identity of the compound?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:30, latezwardjr15
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 22:50, kanerobertrosss2213
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3

Chemistry, 23.06.2019 10:00, kidre96521
State the effect on the concentration of the clo- ion when there is a decrease in the concentration of the oh- ion
Answers: 1
Do you know the correct answer?
A 0.533 g sample of a carbonate salt is added to a flask. The only thing you know is that per formul...
Questions in other subjects:

Social Studies, 17.12.2020 01:00

Arts, 17.12.2020 01:00

Mathematics, 17.12.2020 01:00

Engineering, 17.12.2020 01:00



History, 17.12.2020 01:00

Mathematics, 17.12.2020 01:00



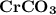
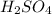 added = 15 × 1 × 10⁻³
added = 15 × 1 × 10⁻³ 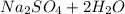
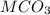 ------>
------> 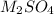 +
+ 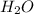 +
+